- Valence Electrons Ca
- Valence Electrons In Calcium Iodide
- Calcium Oxide Valence Electrons
- List Of Valence Electrons For Each Element
Calcium is the third element in the second column of the periodic table. It is classified as an alkaline earth metal. Calcium atoms have 20 electrons and 20 protons. There are 2 valence electrons in the outer shell. Calcium is an important element for life on Earth and is the fifth most abundant element in the Earth's crust. How Many Valence Electrons Are Transferred From The Calcium Atom To Iodine In The Formation Of The Compound Calcium Iodide? How Many Valence Electrons Does The Element Sulfur Have? How Many Valence Electrons Are Found In An Atom Of Aluminum? How Many Valence Electrons Does A Nitrogen Atom Have? Why Is Calcium's Symbol (abbreiviation) Ca?
Click to see full answer
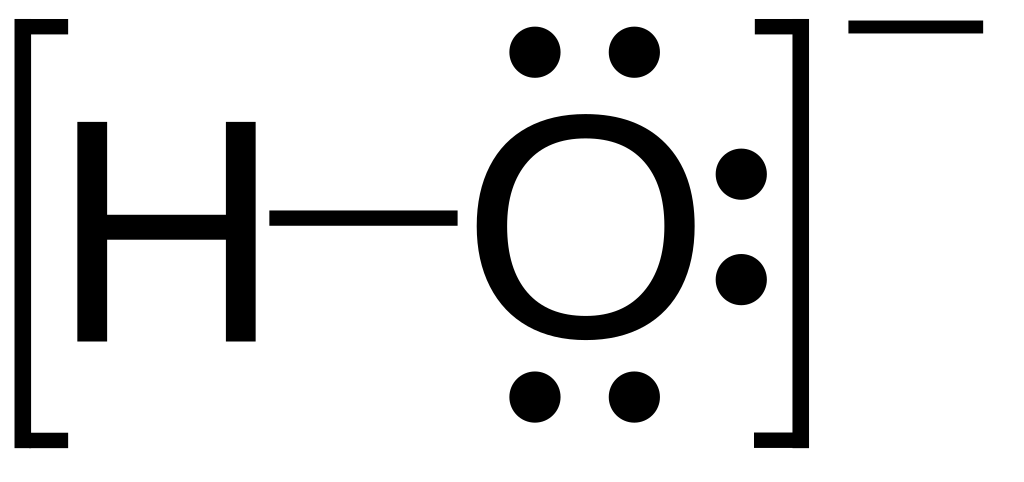
Simply so, what does calcium and fluorine make?
Calcium fluoride is the inorganic compound of the elements calcium and fluorine with the formula CaF2. It is a white insoluble solid. It occurs as the mineral fluorite (also called fluorspar), which is often deeply coloured owing to impurities.
Beside above, how does calcium react with fluorine? Calcium reacts with fluorine to form the compound CaF2. In the reaction, each atom of one of the elements loses two electrons, and each atom of the other element gains one electron. Give Ca 20 neutrons and F 10 neutrons.
Beside this, what bond is formed between calcium and fluorine?
Valence Electrons Ca
Calcium Fluoride is a Quasilinear molecule the bonds are created from the single electrons of calcium and the single electron from fluoride. CaF2 has its electrons contained with in the 3d orbitals and are able to move between dyz and dz2 squared.
Valence Electrons In Calcium Iodide
What happens when potassium and fluorine bond?
1 Answer. A potassium atom has one valence electron in its outermost (fourth) energy level, and fluorine has seven valence electrons in its outermost (second) energy level. In order to obtain eight valence electrons (an octet), the potassium atom will transfer its single valence electron to the fluorine atom.
You can here know about the calcium valence electrons to boost your chemistry knowledge. The article will disclose the various electron valence information of elements. Well, just like the other elements in chemistry Calcium is also a chemical element. The chemical comes from the family of Alkaline earth metals.

- Flerovium Valence Electrons
- Helium Valence Electrons
- Plutonium Valence Electrons
- Lithium Valence Electrons
- Mercury Valence electrons
- Americium Valence Electrons
- Neptunium Valence Electrons
- Oxygen Valence Electrons
- Sodium Valence Electrons
- Magnesium Valence Electrons
- Bismuth Valence electrons
- Aluminum Valence Electrons
- Silicon Valence Electrons
- Beryllium Valence Electrons
- Livermorium Valence Electrons
- Fluorine Valence Electrons
- Radon Valence electrons
- Carbon Valence Electrons
- Xenon Valence Electrons
- Neon Valence Electrons
- Tennessine Valence Electrons
- Antimony Valence Electrons
- Radium Valence Electrons
- Oganesson Valence Electrons
- Iodine Valence Electrons
- Lead Valence electrons
- Tellurium Valence Electrons
- Boron Valence Electrons
- Gold Valence Electrons
- Nobelium Valence Electrons
- Neon Valence Electrons
- Hydrogen Valence Electrons
It’s a very reactive element since it easily reacts in the exposure of oxygen. In appearance, Calcium looks like a grey, silver puffy substance. Calcium is one of those elements which we can easily find in abundance within the earth. Moreover, calcium is also a significant part of the human body structure.
You can find calcium as the core component of human bones etc. It’s one of the most vital naturally occurring chemical elements in human bodies.
Calcium Valence Electrons Dot Diagram
You can definitely refer to the Lewis dot diagram to explore the electron valence of Calcium. The diagram represents the valence electron of Calcium atoms and molecules.
The diagram helps in understanding the bonding structure of atoms and molecules for the valence electrons. We advise you to go through the Dot diagram of Calcium to get in-depth insight.
Valency of Calcium
Calcium Oxide Valence Electrons
The valency of Calcium is always 2+ with its electron configuration as 2,8,8,2. So, it contains 2 electrons in its outer shell. So, in other words, Calcium can either gain or lose 2 electrons. As per the octet rule, calcium will gain stability by gaining or losing 2 electrons.
The valency of a chemical element is an important characteristic of the element. This is therefore the ideal property of the given element to attain stability.
List Of Valence Electrons For Each Element

How many valence electrons does Calcium have?

Furthermore, are generally two rules to figure out the valency of an element. The first rule is the octet rule while the second rule is the duplet rule. You can apply either of the rules to calculate the valency of calcium. Moreover, you can also refer the periodic table to determine the correct valency of Calcium.
